- TOP
- Support & Inquiries
- Technical Glossary
- Silicon and Silicone
Technical Glossary
Silicon and Silicone
[Silicon and Silicone]
Silicon refers to the element Si, atomic number 14. In a highly pure form, silicon is used as a semiconductive material. Silicon is an extremely abundant element in the earth's crust and it can mainly be found in the compound silicon dioxide (SiO2).
Silicone (with an "e" at the end), however is a polymer with a structure as seen in Diagram 3 and is completely different from the elemental silicon.
The term “silicone” was coined by Frederic Stanley Kipping (1863-1949), who laid the foundations of silicon chemistry in England from the late 19th century to the early 20th century. Kipping aimed to create a silicon-based compound that corresponded to the ketone benzophenone. He called the result silicoketone, which was eventually abbreviated to “silicone”.
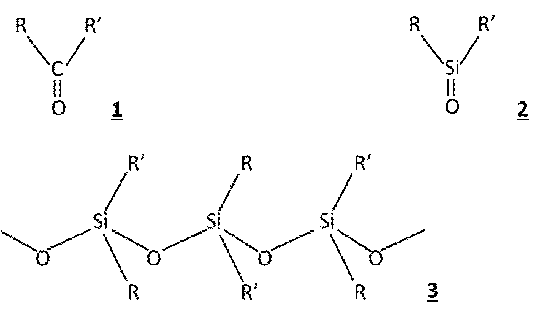
However, unable to form the desired chemical structure (Diagram 2), it is said that, in disappointment, he threw out the resultant oil-like material (Diagram 3) which kept forming in his trials.
After that, a method for mass producing organic silicon compounds was established in the U.S. During World War 2, demand for high quality rubber and resin increased, and silicone polymers with outstanding properties and structures (Diagram 3) were developed quickly to meet that need.
Silicone comes in many shapes and forms, such as oil, gel, rubber, or resin, each of which have different properties. It is used in numerous applications as a lubricant, sealant, and even as tableware. Silicone polymers have excellent cold, heat, weather and electrical resistance, act as absorbers and are non-toxic.
- Customization
- We also develop custom products to meet our customers’ individual needs. Contact us today!
